
LIVTENCITY▼® (maribavir) safety data
An overview of the safety and tolerability profile of LIVTENCITY
A discussion of the outcomes of the phase III SOLSTICE trial featured in this video:
• Dr Sowsan Atabani (Consultant Virologist, UK Health Security Agency and University Hospitals Birmingham)
• Mr Colin Wilson (Consultant Transplant and Hepatobiliary Surgeon, Newcastle Hospitals)
LIVTENCITY is indicated for the treatment of cytomegalovirus (CMV) infection and/or disease that are refractory (with or without resistance) to one or more prior therapies, including ganciclovir, valganciclovir, cidofovir or foscarnet in adult patients who have undergone a haematopoietic stem cell transplant (HSCT) or solid organ transplant (SOT).1
Consideration should be given to official guidance on the appropriate use of antiviral agents.1
Book a meeting
Click this link to discuss the information on this page further with a Takeda representative.
In the SOLSTICE trial, LIVTENCITY delivered double the viraemia clearance (55.7%) compared to investigator-assigned therapy (IAT) (23.9%) – primary endpoint2
Confirmed viraemia clearance* of refractory CMV in all transplants (HSCT and SOT) at Study Week 82
*Plasma CMV DNA <137 IU/mL in 2 consecutive tests ≥5 days apart.2
LIVTENCITY — positive safety profile in post-transplant refractory CMV (with or without resistance)
Fewer treatment emergent adverse events leading to discontinuation than IAT: 13.2% [31/234] of transplant recipients treated with LIVTENCITY experienced a TEAE that led to discontinuation (vs 31.9% [37/116] in those who received investigator assigned therapies)3
LIVTENCITY treatment-related adverse events compared to IAT2,3
In the SOLSTICE trial, the most reported treatment-related adverse event was dysgeusia (35.9% [84/234] vs 0.9% [1/116] receiving IAT) that typically resolved during or after treatment.3
In the SOLSTICE trial, the incidence of neutropenia with LIVTENCITY was lower compared to the IAT arm (1.7% vs 13.8% with IAT; n=4/234 vs n=16/116, respectively)3
In the SOLSTICE trial, transplant patients treated with LIVTENCITY experienced less treatment-related neutropenia vs those receiving valganciclovir/ganciclovir (1.7% vs 25% with valganciclovir/ganciclovir; n=4/234 vs n=14/56, respectively).*2
*Limitations: This study was not powered to detect differences between treatments in this patient subgroup.
In the SOLSTICE trial, the incidence of acute kidney injury (AKI) with LIVTENCITY was lower than the IAT arm (1.7% vs 7.8% with IAT; n=4/234 vs n=9/116)3
In the SOLSTICE trial, transplant patients treated with LIVTENCITY experienced less treatment-related acute kidney injury vs those receiving foscarnet (1.7% vs 19.1% with foscarnet; n=4/234 vs n=9/47).3
*Limitations: This study was not powered to detect differences between treatments in this patient subgroup.
SOLSTICE trial: treatment-emergent adverse events (TEAEs) in ≥10% of subjects in the LIVTENCITY group*2
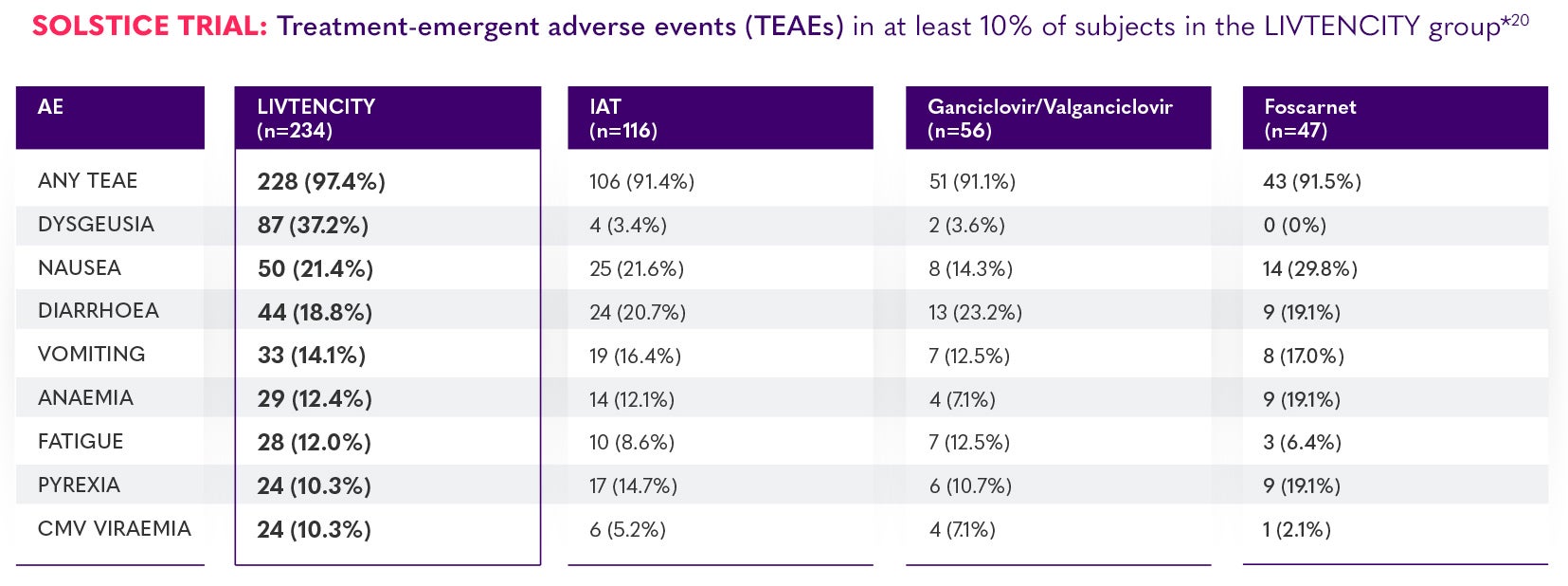
Most commonly-reported TEAE was dysgeusia (37.2% [87/234] receiving LIVTENCITY vs 3.4% [4/116] receiving IAT) that typically resolved during or after treatment.2
*Data are presented as n (%). The cidofovir group was not considered in the application of the 10% cut-off due to low patient numbers (n=6). The on-treatment observation period started at the time of study-assigned treatment initiation through 7 days after the last dose of study-assigned treatment or through 21 days if cidofovir was used, or until the LIVTENCITY rescue treatment initiation or until the non-study CMV treatment initiation, whichever was earlier.2
SOLSTICE trial: Treatment-emergent serious adverse events (TESAEs)*3
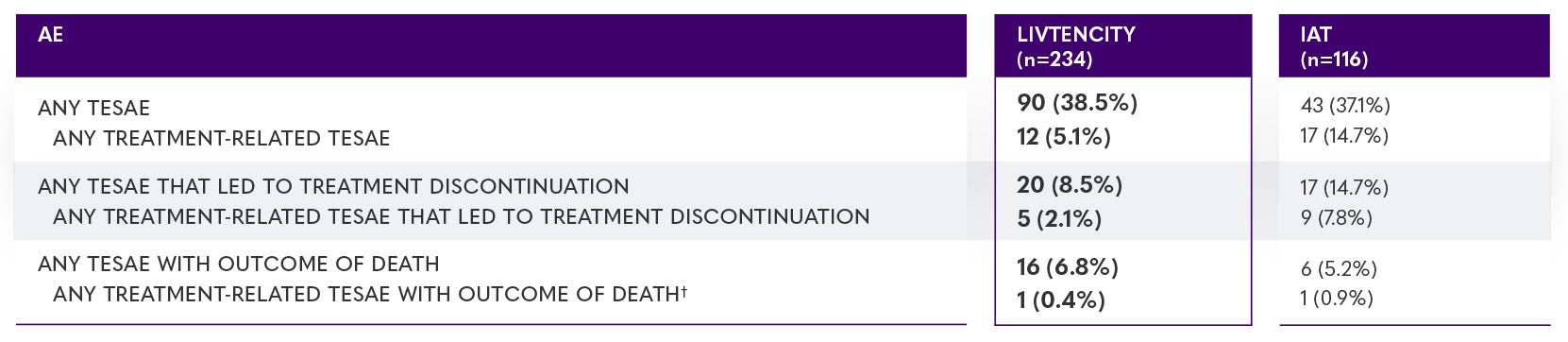
The on-treatment observation period started at the time of study-assigned treatment initiation through 7 days after the last dose of study-assigned treatment or through 21 days if cidofovir was used, or until the maribavir rescue treatment initiation or until the non-study CMV treatment initiation, whichever was earlier.3
*Data are presented as n (%). †In the LIVTENCITY group, there was one treatment-related TESAE of sudden death, potentially due to a cardiac arrhythmia as a result of drug interactions (per investigator). The patient had multiple comorbidities and received concomitant medications known to interact and prolong OT intervals (domperidone, with the risk markedly increased by the introduction of voriconazole and then posaconazole); the event was assessed by the sponsor as being unrelated to LIVTENCITY treatment based on extensive review of the patient's medical history, prior and concomitant medications, and clinical laboratory and electrocardiogram data at screening and baseline visit. In the IAT group, febrile neutropenia, pneumonia, and tuberculosis (one patient) were reported as fatal TESAEs related to valganciclovir.3
SOLSTICE trial: TEAEs leading to treatment discontinuation in ≥2 patients in either treatment group (safety population)*3,4
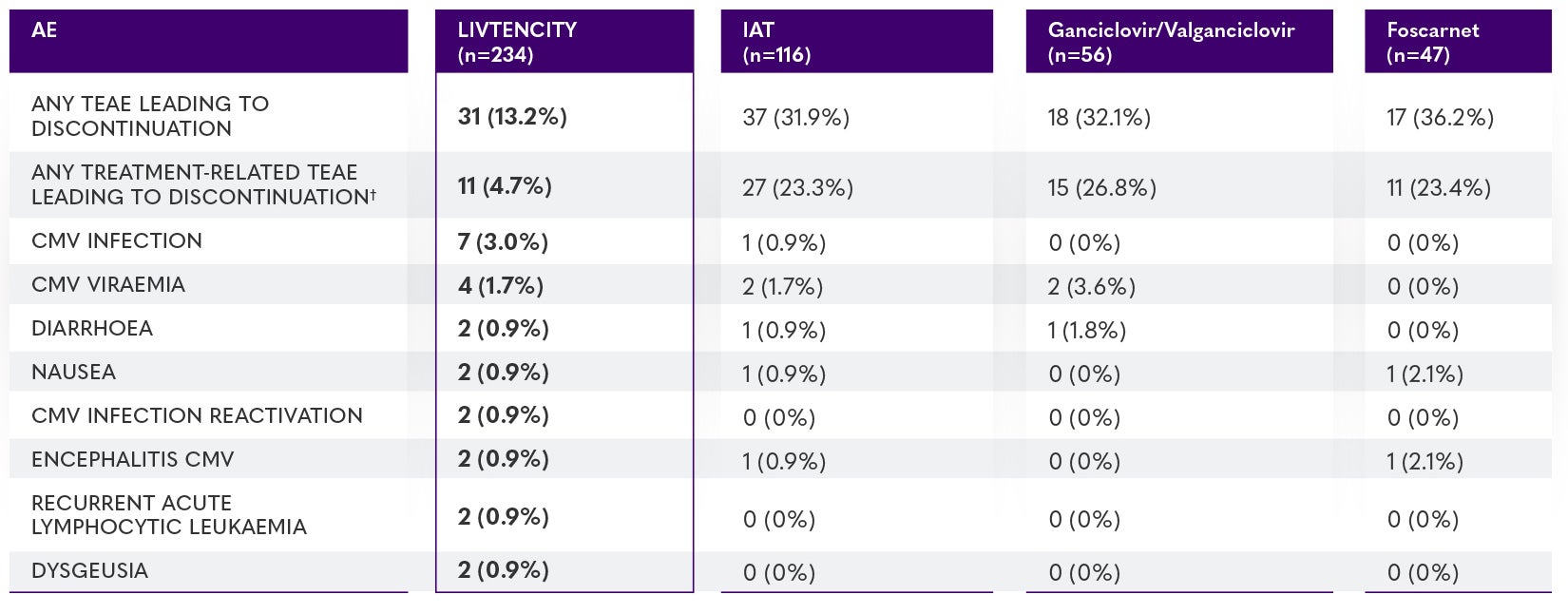
*Data for patients who received cidofovir (n=6) and 1 IAT (n=7) are not presented due to low patient numbers. TEAEs were defined as any adverse event occurring during the on-treatment observation phase. The on-treatment observation phase was from the time of study-assigned treatment initiation through 7 days after the last dose of study-assigned treatment (21 days for cidofovir), or until the LIVTENCITY rescue treatment initiation or the non-study CMV treatment initiation, whichever was earlier.4
†In 2 patients in either treatment group (safety population).4
SOLSTICE trial: summary of treatment emergent adverse events (observation period of rescue arm)*5
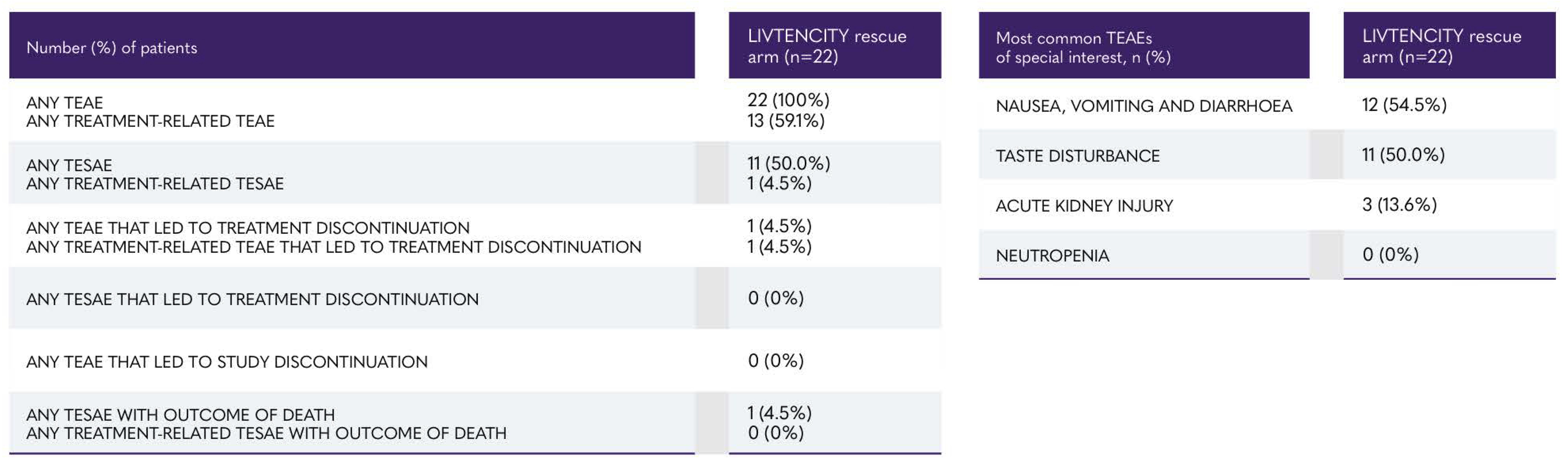
*The on-rescue treatment observation period started at the time of LIVTENCITY rescue treatment initiation through 7 days after the last dose of rescue treatment, or until the non-study CMY treatment initiation, whichever was earlier. TEAEs were defined as any adverse event occurring during the on-rescue treatment observation period.5
Adverse reactions identified with LIVTENCITY 1
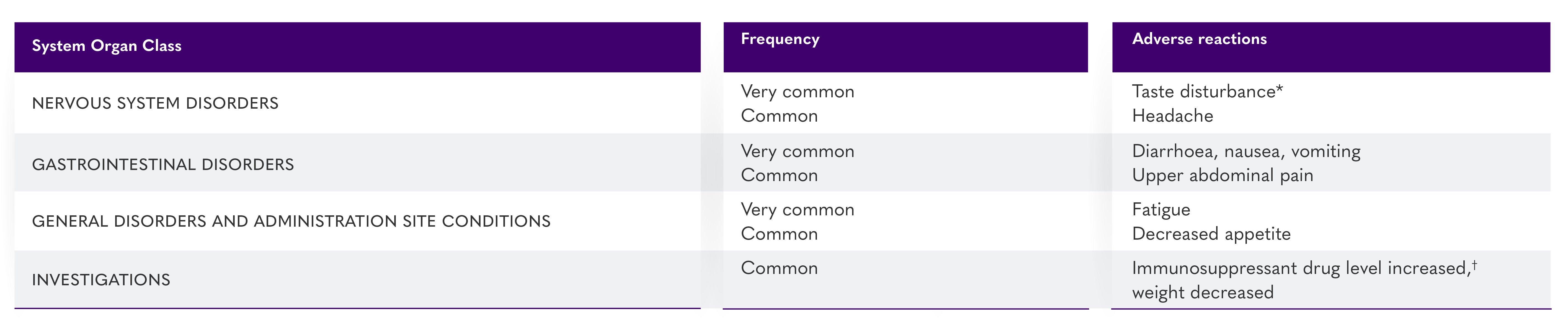
*Taste disturbance (comprised of the reported preferred terms ageusia, dysgeusia, hypogeusia and taste disorder) occurred in 46% of patients treated with LIVTENCITY. These events rarely led to discontinuation of LIVTENCITY (0.9%) and, for most patients, resolved while patients remained on therapy (37%) or within a median of 7 days (Kaplan-Meier estimate, 95% Cl: 4–8 days) after treatment discontinuation.1
†Immunosuppressant drug level increase (comprised of the preferred terms immunosuppressant drug level increased and drug level increased) occurred in 9% of patients treated with LIVTENCITY. LIVTENCITY has the potential to increase the drug concentrations of immunosuppressants that are CYP3A and/or P-gp substrates with narrow therapeutic ranges (including tacrolimus, cyclosporine, sirolimus and everolimus).1
For information on the safety profile of LIVTENCITY, its special warnings and precautions for use in relation to immunosuppressants and certain medicinal products, as well as the contraindications and drug-drug interactions, please consult the LIVTENCITY Summary of Product Characteristics
IAT = one or a combination of ganciclovir, valganciclovir, foscarnet, or cidofovir.2
Refractory CMV = failure to achieve >1 log10 decrease in CMV DNA level in whole blood or plasma after a 14-day or longer treatment period with intravenous (IV) ganciclovir/oral valganciclovir; IV foscarnet, or IV cidofovir.2
AE = adverse event; CNS = central nervous system; CMV = cytomegalovirus; Gl = gastrointestinal; HIV = human immunodeficiency virus;
HMG-CoA = 3-hydroxy-3-methyl-glutaryl-coenzyme A; IAT = investigator-assigned therapy; P-gp = P-glycoprotein; SD = standard deviation; TEAE = treatment-emergent adverse event.
References:
- LIVTENCITY UK Summaries of Product Characteristics [GB & NI].
- Avery RK, et al. Clin Infect Dis. 2022;75(4):690–701.
- Avery RK, et al. Clin Infect Dis. 2022;75(4):690–701. (Supplementary data).
- Alexander BO, et al. Poster 467 Presented at the Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR. April 23-26, 2022. Salt Lake City, UT.
- Pereira M, et al. Efficacy and safety of Maribavir as a rescue treatment for investigator assigned therapy in transplant recipients with refractory or resistant cytomegalovirus infections in the SOLSTICE study: phase 3 trial results. In: The 2021 IDWeek Virtual Conference; 2021. Oral Abstract 21.
Click on the links below to learn more:
Current unmet needs in the management of CMV post-transplant


