
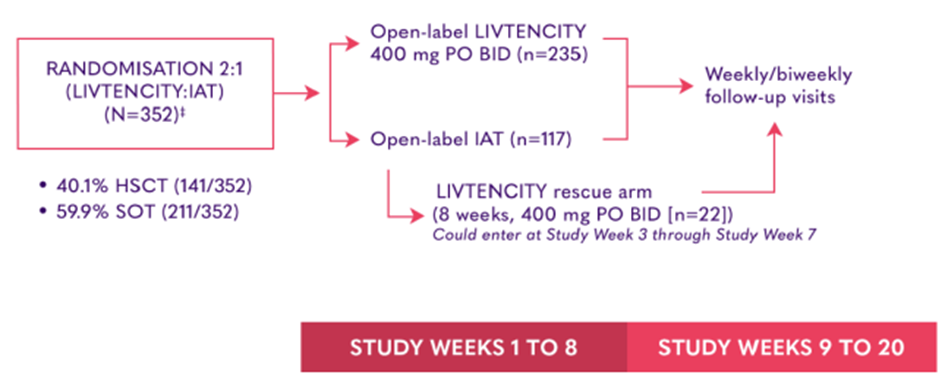
SOLSTICE study design
LIVTENCITY▼® (maribavir) is indicated for the treatment of cytomegalovirus (CMV) infection and/or disease that are refractory (with or without resistance) to one or more prior therapies, including ganciclovir, valganciclovir, cidofovir or foscarnet in adult patients who have undergone a haematopoietic stem cell transplant (HSCT) or solid organ transplant (SOT).1
Consideration should be given to official guidance on the appropriate use of antiviral agents.1
SOLSTICE: LIVTENCITY (maribavir) vs IAT in transplant recipients with refractory CMV infection2
The SOLSTICE (TAK-620-303) trial was a multicentre, randomised, open-label, active- controlled trial, assessing efficacy and safety of LIVTENCITY (maribavir) compared to IAT in HSCT (n=141) and SOT (n=211) recipients with CMV infection refractory to one or a combination of the IATs: ganciclovir, valganciclovir, foscarnet, or cidofovir2
SOLSTICE primary endpoint2
Confirmed CMV viraemia clearance (plasma CMV DNA <137 IU/mL in 2 consecutive tests ≥5 days apart) compared to IAT at the end of Study Week 8.2
Safety endpoints included treatment-emergent adverse events (TEAEs) and serious TEAEs (TESAEs).
SOLSTICE key secondary endpoint2
Achievement of CMV viraemia clearance* and symptom control† at the end of Study Week 8, maintained through Study Week 16.
*CMV viraemia clearance = plasma CMV DNA < lower limit of quantification (i.e. <137 IU/mL) in 2 consecutive tests ≥5 days apart.2
†CMV infection symptom control was defined as resolution or improvement of tissue-invasive disease or CMV syndrome for symptomatic patients at baseline, or no new symptoms for patients who were asymptomatic at baseline.2
‡Additional stratification by baseline CMV DNA level (high, intermediate, and low).2
*Based on most recent transplant type. Those classed as “multiple” had multiple organs transplanted at once.2
†The denominator is the number of patients who received solid organ transplant within each treatment arm.2
‡There was 1 (1.1%) autologous haematopoietic stem cell transplant in the LIVTENCITY group.2
Inclusion criteria
- HSCT or SOT recipient aged ≥12 years*
- ≥2730 IU/mL (whole blood) or ≥910 IU/mL (plasma)
- CMV infection refractory to most recently administered anti-CMV agent
- Screening eGFR >30 mL/min/1.73m2, ANC ≥1000/mm3, platelet count ≥25,000/mm3
Exclusion criteria
- Resistant or refractory CMV infection due to inadequate adherence to prior anti-CMV treatment
- CMV disease with central nervous system involvement or retinitis
- Concomitant need for leflunomide, letermovir, or artesunate
*Although the inclusion criteria was ≥12 years, all patients were >18 years old.2
The SOLSTICE study has the following limitations:2
- Open-label design – a blinded study was not possible due to the need for individualised IAT drug selection, dosing adjustments and the different administration routes for IAT compared with LIVTENCITY
- A numerically greater response for LIVTENCITY versus IAT was observed in patients with refractory CMV without genotyped resistance, in alignment with overall study results. However, the study was not powered to detect differences between treatments in this patient subgroup
- Patients were not stratified by refractory or resistant CMV at randomisation. Therefore, more patients with refractory-only CMV were included in the LIVTENCITY group
- A higher study discontinuation rate before Week 8 in the IAT group was observed. Discontinuations were primarily due to adverse events and toxicities known to be associated with available anti-CMV agents. Sensitivity analyses were performed to address this potential bias
- Patients with residual CMV at the end of treatment (Week 8) may have needed an alternative treatment during follow-up due to the study-specified treatment duration. These patients were therefore considered non-responders to the key secondary endpoint
IAT = one or a combination of ganciclovir, valganciclovir, foscarnet, or cidofovir.2
Refractory CMV = failure to achieve >1 log10 decrease in CMV DNA level in whole blood or plasma after a 14-day or longer treatment period with intravenous (IV) ganciclovir/oral valganciclovir; IV foscarnet, or IV cidofovir.2
ANC = absolute neutrophil count; BID = twice daily; CMV = cytomegalovirus; eGFR = estimated glomerular filtration rate; HSCT = haematopoietic stem cell transplant; IAT = investigator-assigned therapy; IV = intravenous; PO = by mouth; SOT = solid organ transplant
References:
- LIVTENCITY UK Summaries of Product Characteristics [GB & NI].
- Avery RK, et al. Clin Infect Dis. 2022;75(4):690–701.
- Avery RK, et al. Clin Infect Dis. 2022;75(4):690–701. (Supplementary data).


