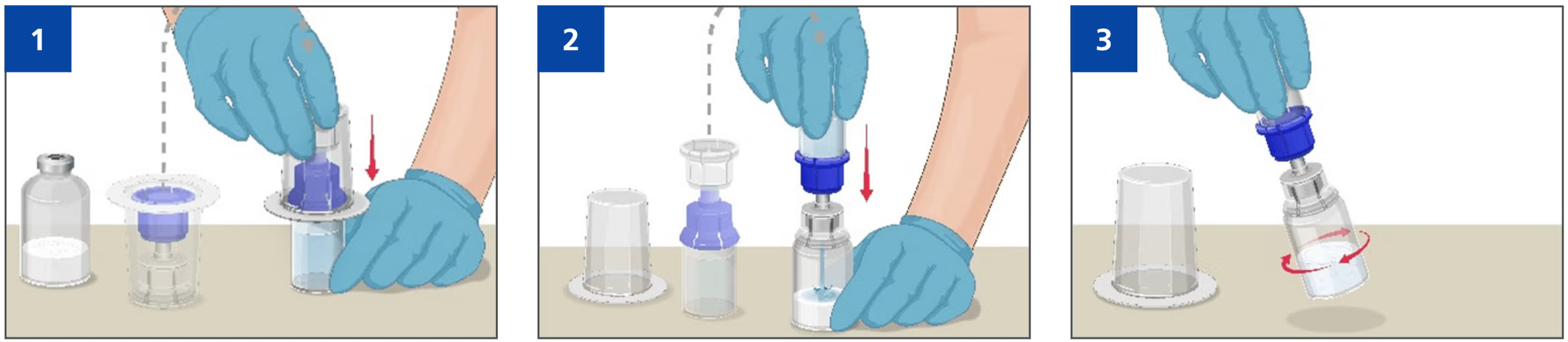
Prothromplex TOTAL 500 IU Mix2Vial® presentation is a simplified needle-free reconstitution vs the previous Prothromplex TOTAL presentation, which may reduce the likelihood of needle stick injuries1,2
Preparation
Monitoring of international normalised ratio (INR) during treatment is mandatory.
Refer to the Summary of Product Characteristics before use.
For reconstitution only the enclosed reconstitution set should be used
- Remove the protective caps from the powder vial and the solvent vial.
- Disinfect each stopper with a separate sterile alcohol swab (or other suitable sterile solution) by wiping the stopper for several seconds.
- Allow the rubber stopper to dry.
- Place the vials on a flat surface Open the Mix2Vial® device package but do not remove from packaging
Mixing
- Turn the package with the Mix2Vial® device upside down and place it over the top of the solvent vial.
- Firmly insert the blue plastic spike of the device into the centre of the solvent vial stopper.
- Turn the solvent vial over, place it on top of the vial containing Prothromplex TOTAL powder, then gently and continuously swirl the connected vials until dissolved or allow the reconstituted product to stand for 5 minutes then gently swirl to ensure that the powder is completely dissolved.
- Do not shake. Shaking will adversely affect the product.
- Do not refrigerate after reconstitution Do not use if the vacuum has been lost and the solvent does not flow into the Prothromplex TOTAL vial.
Withdrawal
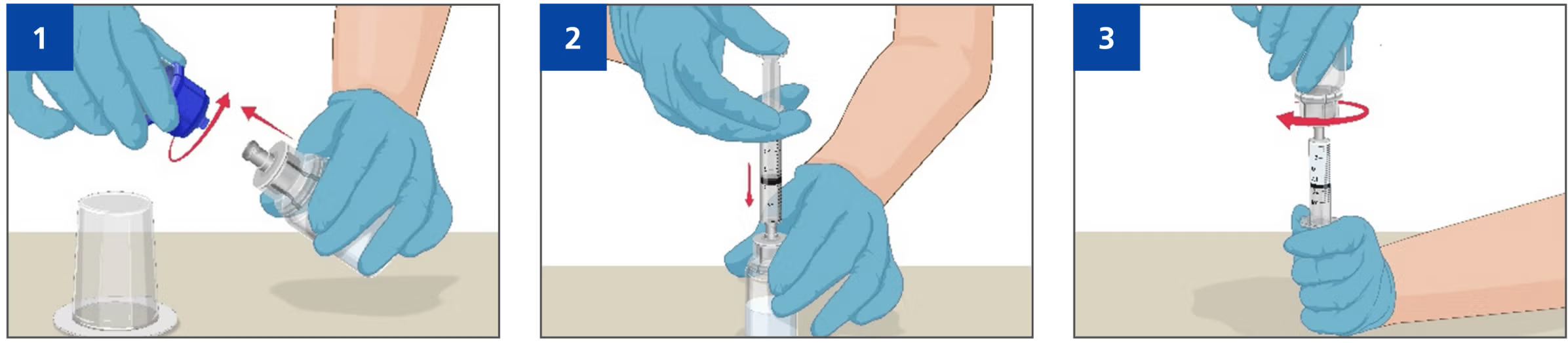
- Disconnect the two sides of the Mix2Vial® device from each other and place the Prothromplex TOTAL vial with the clear plastic side on a flat work surface.
- Draw air into an empty, sterile disposable plastic syringe, connect to the clear plastic connector and push all the air from the syringe into the vial.
- The amount of air should equal the amount of reconstituted Prothromplex TOTAL that you will withdraw from the vial Flip connected syringe and Prothromplex TOTAL vial, so the vial is on top.
- Be sure to keep the syringe plunger pressed in Draw the Prothromplex TOTAL into the syringe by pulling plunger back slowly, when ready disconnect the syringe.
 For full details please see the Summary of Product Characteristics
For full details please see the Summary of Product Characteristics
Prothromplex TOTAL 500 IU allows ease of dosing, increasing Healthcare Professional (HCP) and health system choice within existing clinical protocols based on multiples of 500 IU prothrombin complex concentrates (PCCs)3,4
Bleeding and perioperative prophylaxis of bleeding during vitamin K antagonist treatment
If Prothromplex TOTAL administration is based on the INR measurement, the dose will depend on the INR before treatment and the targeted INR.1

The dosage and duration of the substitution therapy depend on the severity of the coagulation disorder, on the location and extent of the bleeding and on the patient’s clinical condition. Dosage and frequency of administration must be calculated on an individual patient basis. Individual dosage requirements can only be identified on the basis of regular determinations of the individual plasma levels of the coagulation factors of interest or on the global test of the prothrombin complex level (e.g., Quick's time value, INR, prothrombin time) and continuous monitoring of the patient’s clinical condition.1
Monitoring of INR during treatment is mandatory.1
Bleeding and perioperative prophylaxis in congenital deficiency of any of the vitamin K-dependent coagulation factors when specific coagulation factor product is not available:1
Required units = body weight (kg) x desired factor X rise (IU/ml) x 60
Hereditary combined vitamin K-dependent clotting factors deficiency is a rare inherited coagulation defect that forms part of a wider group of rare disorders named Familial Multiple Coagulation Factor Deficiencies.5
Replacement therapy with Prothromplex TOTAL, may result in the formation of circulating antibodies inhibiting one or more of the human prothrombin complex factors. If such inhibitors occur, the condition will manifest itself as a poor clinical response.1
Allergic or anaphylactic-type reactions have been commonly observed.1
Increase in body temperature has been commonly observed.1
There is a risk of thromboembolic episodes, following the administration of human prothrombin complex.1
For full details please see the Summary of Product Characteristics
- Prothromplex TOTAL 500 IU SmPC. Available from: https://www.medicines.org.uk/emc/product/15237 (accessed June 2024)
- Takeda Data on File (EXA/GB/PROT/0003);
- Eichinger S. Hematology Am Soc Hematol Educ Program. 2016;1:605-611;
- Milling T and Pollack CV. Am J Emerg Med. 2020;38(9):1890-1903;
- Napolitano, M. et al. Orphanet J Rare Dis. 2010;5:21
Browse the Prothromplex hub for more product information
View resources
You might be interested in
download
Download the Prothromplex TOTAL® (human prothrombin complex) Implementation Checklist. This document will help you track and manage the necessary steps for incorporating Prothromplex TOTAL® (human prothrombin complex) into your prescribing protocols.
download

Download the Prothromplex® TOTAL (human prothrombin complex) 500 IU Formulary Support Document. This document provides relevant information to support formulary applications for Prothromplex® TOTAL (human prothrombin complex) 500 IU.
download

Access and download the Prothromplex® TOTAL (human prothrombin complex) 500 IU Guideline Support Pack. This contains information to support local guideline/protocol development.
download

Please click here to download the Prothromplex® TOTAL (human prothrombin complex) 500 IU Dosing Calculator. This Excel document allows you to calculate the dose requirements per patient’s weight and initial international normalised ratio (INR)
download

Download the Prothromplex® TOTAL (human prothrombin complex) 500 IU Reconstitution and Dosing Guide. This document will guide you through the Prothromplex® TOTAL (human prothrombin complex) 500IU reconstitution process and how to calculate the appropriate dose for individual patients.


 I am a healthcare professional in the UK
I am a healthcare professional in the UK
 I am a patient or member of the public
I am a patient or member of the public