LIVTENCITY▼® (maribavir) efficacy data
LIVTENCITY (maribavir) is indicated for the treatment of cytomegalovirus (CMV) infection and/or disease that are refractory (with or without resistance) to one or more prior therapies, including ganciclovir, valganciclovir, cidofovir or foscarnet in adult patients who have undergone a haematopoietic stem cell transplant (HSCT) or solid organ transplant (SOT).1
Consideration should be given to official guidance on the appropriate use of antiviral agents.1
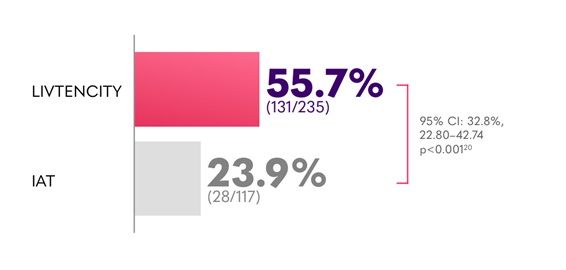
In the pivotal Phase 3 trial, LIVTENCITY delivered viraemia clearance in twice the proportion of patients (55.7%) compared to investigator-assigned therapy (IAT) (23.9%) - primary endpoint2
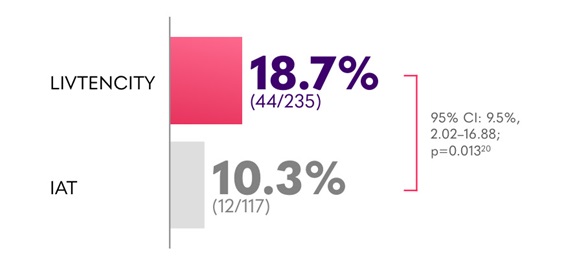
In the pivotal Phase 3 trial, LIVTENCITY delivered viraemia clearance of 18.7% at Study Week 8 maintained through Week 16 - almost double the proportion of patients compared with IAT (10.3%) - key secondary endpoint2
Confirmed viraemia clearance* of refractory CMV in all transplants (HSCT and SOT) at Study Week 82
Confirmed CMV viraemia clearance and symptom control* in all transplants (HSCT and SOT) at the end of Study Week 8, maintained through Study Week 162
*Plasma CMV DNA <137 IU/mL in 2 consecutive tests ≥5 days apart.2
†CMV infection symptom control was defined as resolution or improvement of tissue-invasive disease or CMV syndrome for symptomatic patients at baseline, or no new symptoms for patients who were asymptomatic at baseline.2
LIVTENCITY - safety profile in post-transplant refractory CMV (with or without resistance)
- In the SOLSTICE trial, the most reported treatment-related adverse event was dysgeusia that typically resolved during or after treatment (35.9% [84/234] receiving LIVTENCITY vs 0.9% [1/116] receiving investigator-initiated therapy)3
- Transplant recipients treated with LIVTENCITY experienced less treatment-related neutropenia vs those receiving valganciclovir/ganciclovir (1.7% receiving LIVTENCITY vs 25% receiving valganciclovir/ganciclovir)2
- Transplant patients treated with LIVTENCITY experienced less treatment-related acute kidney injury vs those receiving foscarnet (1.7% receiving LIVTENCITY vs 19.1% receiving foscarnet)2
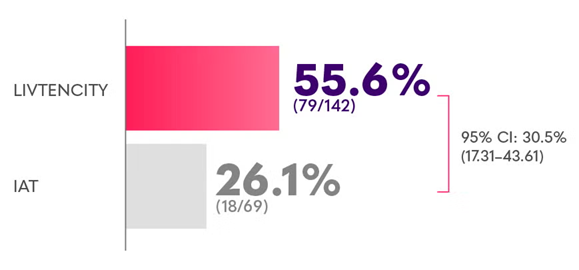
In subgroup analyses of the pivotal Phase 3 trial, viraemia clearance was observed in SOT recipients2-5
Confirmed viraemia clearance in a subgroup analysis of SOT recipients with refractory CMV at Study Week 8*2,4

Confirmed CMV viraemia clearance and symptom control at the end of Study Week 8, maintained through Study Week 16 in a subgroup analysis of SOT recipients with refractory CMV3
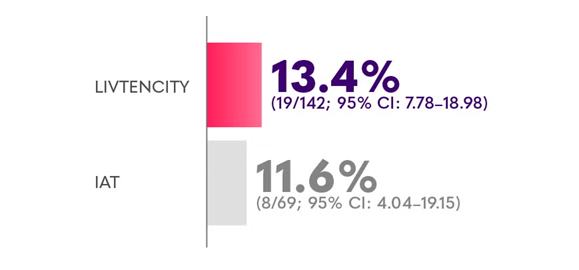
*Limitations: This study was not powered to detect differences between treatments in this patient subgroup.2
SOLSTICE primary endpoint: subgroup analysis of efficacy by organ type


In a subgroup analysis of heart transplant recipients with refractory CMV receiving LIVTENCITY (6/14; vs 11.1% [1/9] on IAT)4
95% Cl: 30.7% (-1.72–63.2)


In a subgroup analysis of lung transplant recipients with refractory CMV receiving LIVTENCITY (19/40; vs 13.6% [3/22] on IAT)4
95% Cl: 38.2% (16.9–59.5)


In a subgroup analysis of kidney transplant recipients with refractory CMV receiving LIVTENCITY (44/74; vs 34.4% [11/32] on IAT)4
95% Cl: 26.7% (7.5–45.9)


In a subgroup analysis of liver transplant recipients with refractory CMV receiving LIVTENCITY vs 0/1 on IAT5
Note: this subgroup analysis has been calculated from low patient numbers and the two treatment groups are not equally matched; therefore, it is insufficient to show a difference in treatment effect.
In subgroup analyses of the pivotal Phase 3 trial, viraemia clearance was observed in HSCT recipients2,6

Confirmed viraemia clearance in a subgroup analysis (randomised population) of HSCT recipients with refractory CMV at Study Week 8*2
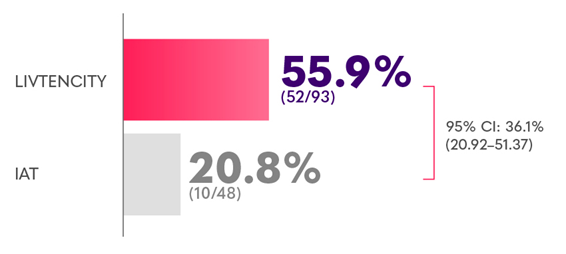

Confirmed CMV viraemia clearance and symptom control† at the end of Study Week 8, maintained through Study Week 16 in a subgroup analysis (randomised population) of HSCT recipients with refractory CMV6
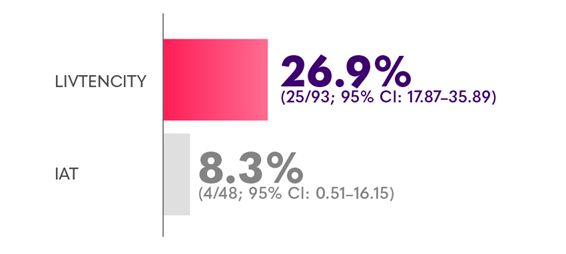
*Limitations: This study was not powered to detect differences between treatments in this patient subgroup.2
†CMV infection symptom control was defined as resolution or improvement of tissue-invasive disease or CMV syndrome for symptomatic patients at baseline, or no new symptoms for patients who were asymptomatic at baseline.2
SOLSTICE trial: rescue arm2
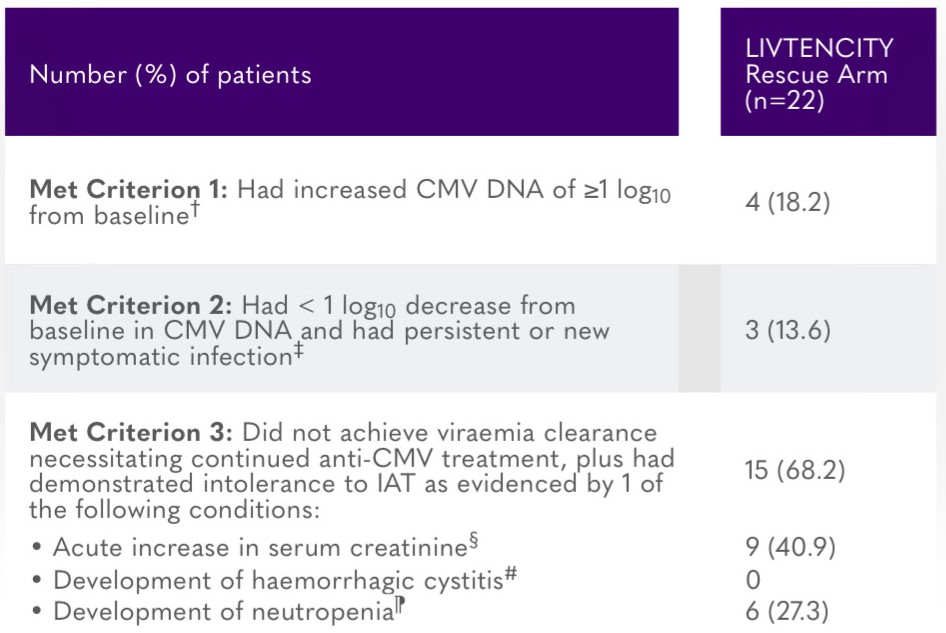
Summary of HSCT and SOT recipients randomised to IAT meeting criteria for entry into LIVTENCITY rescue arm*7
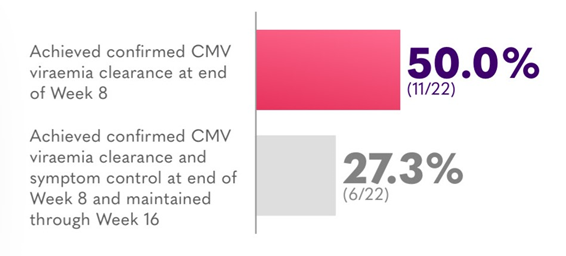
HSCT and SOT recipients in rescue arm achieving confirmed CMV viraemia clearance**7
*All patients who entered the LIVTENCITY rescue arm had demonstrated persistence of CMV viraemia despite treatment with IAT for at least 3 weeks. All patients entered the rescue arm between study Weeks 3–7, except for 1 patient who entered between Weeks 7–8.7
†Whole blood or plasma CMV DNA levels were measured by local or central specialty laboratory qPCR assay.7
‡Patient had to meet 2 criteria: (i) whole blood or plasma CMV DNA had decreased <1 log10 from baseline as measured by local or speciality laboratory qPCR assay, and (ii) the presenting tissue-invasive CMV disease for symptomatic patients had not improved/worsened, or patient was asymptomatic at baseline and developed tissue-invasive disease.7
§At least 50% increase from baseline value, attributed to treatment toxicity (e.g. cidofovir, foscarnet).7
#When on treatment with cidofovir or foscarnet.7
¶Absolute neutrophil count <500/mm3 when on treatment with valganciclovir/ganciclovir.7
**Included all patients who entered the rescue arm and received any dose of LIVTENCITY as rescue therapy.7
Confirmed viraemia clearance = plasma CMV DNA < lower limit of quantification (i.e. <137 IU/mL) in 2 consecutive tests ≥5 days apart.2
IAT = one or a combination of ganciclovir, valganciclovir, foscarnet, or cidofovir.2
Refractory CMV = failure to achieve >1 log10 decrease in CMV DNA level in whole blood or plasma after a 14-day or longer treatment period with intravenous (IV) ganciclovir/oral valganciclovir; IV foscarnet, or IV cidofovir.2
Cl = confidence interval; CMV = cytomegalovirus; HSCT = haematopoietic stem cell transplant; IAT = investigator-assigned therapy; qPCR = quantitative polymerase chain reaction; SOT = solid organ transplant.
References:
- LIVTENCITY UK Summaries of Product Characteristics.
- Avery RK, et al. Clin Infect Dis. 2022;75(4):690–701.
- Data on File for Maribavir for SOT recipients. EXA/GB/MARI/0029.
- Avery RK, et al. Am J Transplant. 2021;21(suppl. 3).
- Data on File for Maribavir for 100% CMV clearance in LT patients. EXA/GB/MARI/0030.
- Data on File for Maribavir for HCT recipients. EXA/GB/MARI/0028.
- Pereira M, et al. Efficacy and safety of Maribavir as a rescue treatment for investigator assigned therapy in transplant recipients with refractory or resistant cytomegalovirus infections in the SOLSTICE study: phase 3 trial results. In: The 2021 IDWeek Virtual Conference; 2021. Oral Abstract 21.
You might be interested in
download

This concise guide to LIVTENCITY® (maribavir) is designed for clarity and ease of use. It summarises key insights from the Phase 3 SOLSTICE trial, including efficacy outcomes, safety data, and practical dosing guidance.
article

A summary of the safety profile of LIVTENCITY, including adverse event data and tolerability findings from clinical studies.
article

CMV is one of the most common infections experienced by transplant recipients
article

An overview of the pivotal Phase 3 SOLSTICE trial, outlining the study design, patient population, and methodology that underpin the clinical evidence for LIVTENCITY.


 I am a healthcare professional in the UK
I am a healthcare professional in the UK
 I am a patient or member of the public
I am a patient or member of the public





 1 minute
1 minute









